Bond Parameters
Bond Parameters: Overview
This Topic covers sub-topics such as Bond Order, Bond Length, Bond Enthalpy, Bond Angle, Bond Parameters, Dipole Moment of Polyatomic Molecules, Dipole Moment Comparison for NH3 and NF3 and, Bond Enthalpy for Molecules containing Multiple Bonds
Important Questions on Bond Parameters
Bond formation is :
Which of the following statements are true.
(i) In the structure of the bond is shorter than the bond
(ii) All the bonds in are not equivalent.
(iii) is more reactive than
bond length is maximum in
The $\mathrm{H}-\mathrm{O}-\mathrm{H}$ angle in water molecule is about
The bond orders of and are:
The dipole moment of the given molecules are such that :
What will be correct order of halogen atoms for the increasing order of bond angle in molecule.
Select the correct option regarding bond length.
Which of them has minimum value of where is bond angle of given species?
Which of the following has least bond angle = halogen?
Molecule with non-zero dipole moments is/are
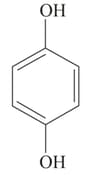
The order of increasing bond length of is :
Which one of the following statements is correct for
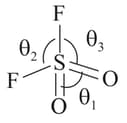
In which of the following bond is strongest among the given four compounds? central atom
Among and which statement is not correct?
The correct order in which the bond length increases in the following:
The correct order of decreasing bond angle is :
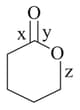 Correct order of Bond length is:
Correct order of Bond length is:
Which of the following would have a permanent dipole moment :-
Correct order of bond lengths is :-
